annealing发表评论(0)编辑词条
Annealing
Annealing, in metallurgy and materials science, is a heat treatment wherein a material is altered, causing changes in its properties such as strength and hardness. It is a process that produces conditions by heating to above the re-crystallization temperature and maintaining a suitable temperature, and then cooling. Annealing is used to induce ductility, soften material, relieve internal stresses, refine the structure by making it homogeneous, and improve cold working properties.
In the cases of copper, steel, silver, and brass this process is performed by substantially heating the material (generally until glowing) for a while and allowing it to cool slowly. In this fashion the metal is softened and prepared for further work such as shaping, stamping, or forming. It also presents no problem with decarburization.
Thermodynamics of annealing
Annealing occurs by the diffusion of atoms within a solid material, so that the material progresses towards its equilibrium state. Heat is needed to increase the rate of diffusion by providing the energy needed to break bonds. The movement of atoms has the effect of redistributing and destroying the dislocations in metals and (to a lesser extent) in ceramics. This alteration in dislocations allows metals to deform more easily, so increases their ductility.
The amount of process-initiating Gibbs free energy in a deformed metal is also reduced by the annealing process. In practice and industry, this reduction of Gibbs free energy is termed "stress relief".
The relief of internal stresses is a thermodynamically spontaneous process; however, at room temperatures, it is a very slow process. The high temperatures at which the annealing process occurs serve to accelerate this process.
The reaction facilitating the return of the cold-worked metal to its stress-free state has many reaction pathways, mostly involving the elimination of lattice vacancy gradients within the body of the metal. The creation of lattice vacancies are governed by the Arrhenius equation, and the migration/diffusion of lattice vacancies are governed by Fick’s laws of diffusion.[1]
Mechanical properties, such as hardness and ductility, change as dislocations are eliminated and the metal's crystal lattice is altered. On heating at specific temperature and cooling it is possible to bring the atom at the right lattice site and new grain growth can improve the mechanical properties.
Stages of annealing
There are three stages in the annealing process, with the first being the recovery phase, which results in softening of the metal through removal of crystal defects (the primary type of which is the linear defect called a dislocation) and the internal stresses which they cause. Recovery phase covers all annealing phenomena that occur before the appearance of new strain-free grains.[2] The second phase is recrystallization, where new strain-free grains nucleate and grow to replace those deformed by internal stresses.[2] If annealing is allowed to continue once recrystallization has been completed, grain growth will occur, in which the microstructure starts to coarsen and may cause the metal to have less than satisfactory mechanical properties.
Annealing in a controlled atmosphere
The low temperature of annealing (about 50 °F above C3 line) may result in oxidation of the metal’s surface, resulting in scale. If scale is to be avoided, annealing is carried out in an oxygen-, carbon-, and nitrogen-free atmosphere (to avoid oxidation, carburization, and nitriding respectively) such as endothermic gas (a mixture of carbon monoxide, hydrogen gas, and nitrogen[clarification needed]).
The magnetic properties of mu-metal (Espey cores) are introduced by annealing the alloy in a hydrogen atmosphere.
Setup and Equipment
Typically, large ovens are used for the annealing process. The inside of the oven is large enough to place the workpiece in a position to receive maximum exposure to the circulating heated air. For high volume process annealing, gas fired conveyor furnaces are often used. For large workpieces or high quantity parts Car-bottom furnaces will be used in order to move the parts in and out with ease. Once the annealing process has been successfully completed, the workpieces are sometimes left in the oven in order for the parts to have a controled cooling process. While some workpieces are left in the oven to cool in a controled fashion, other materials and alloys are removed from the oven. After being removed from the oven, the workpieces are often quickly cooled off in a process known as quench hardening. Some typical methods of quench hardening materials involve the use of media such as air, water, oil, or salt.
Diffusion annealing of semiconductors
In the semiconductor industry, silicon wafers are annealed, so that dopant atoms, usually boron, phosphorus or arsenic, can diffuse into substitutional positions in the crystal lattice, resulting in drastic changes in the electrical properties of the semiconducting material.
Specialized annealing cycles
Normalization
Normalization is an annealing process in which a metal is cooled in air after heating.
This process is typically confined to hardenable steel. It is used to refine grains which have been deformed through cold work, and can improve ductility and toughness of the steel. It involves heating the steel to just above its upper critical point. It is soaked for a short period then allowed to cool in air. Small grains are formed which give a much harder and tougher metal with normal tensile strength and not the maximum ductility achieved by annealing.
Process annealing
Process annealing, also called "intermediate annealing", "subcritical annealing", or "in-process annealing", is a heat treatment cycle that restores some of the ductility to a work piece allowing it be worked further without breaking. Ductility is important in shaping and creating a more refined piece of work through processes such as rolling, drawing, forging, spinning, extruding and heading. The piece is heated to a temperature typically below the austenizing temperature, and held there long enough to relieve stresses in the metal. The piece is finally cooled slowly to room temperature. It is then ready again for additional cold working. This can also be used to ensure there is reduced risk of distortion of the work piece during machining, welding, or further heat treatment cycles.
The temperature range for process annealing ranges from 500 °F to 1400 °F, depending on the alloy in question.
Full anneal
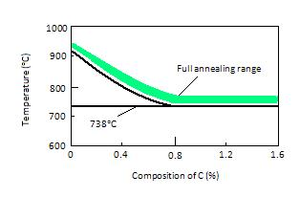
Full annealing temperature rangesA full anneal typically results in the second most ductile state a metal can assume for metal alloy. It creates an entirely new homogeneous and uniform structure with good dynamic properties. To perform a full anneal, a metal is heated to its annealing point (about 50°C above the austenic temperature as graph shows) and held for sufficient time to allow the material to fully austenitize, to form austenite or austenite-cementite grain structure. The material is then allowed to cool slowly so that the equilibrium microstructure is obtained. In some cases this means the material is allowed to air cool. In other cases the material is allowed to furnace cool. The details of the process depend on the type of metal and the precise alloy involved. In any case the result is a more ductile material that has greater stretch ratio and reduction of area properties but a lower yield strength and a lower tensile strength. This process is also called LP annealing for lamellar pearlite in the steel industry as opposed to a process anneal which does not specify a microstructure and only has the goal of softening the material. Often material that is annealed will be machined and then be followed by further heat treatment to obtain the final desired properties.
Short cycle anneal
Short cycle annealing is used for turning normal ferrite into malleable ferrite. It consists of heating, cooling, and then heating again from 4 to 8 hours.
退火
退火 :将金属缓慢加热到一定温度,保持足够时间,然后以适宜速度冷却(通常是缓慢冷却,有时是控制冷却)的一种金属热处理[1]工艺。目的是使经过铸造、锻轧、焊接或切削加工的材料或工件软化,改善塑性和韧性,使化学成分均匀化,去除残余应力,或得到预期的物理性能。退火工艺随目的之不同而有多种,如重结晶退火、等温退火、均匀化退火、球化退火、去除应力退火、再结晶退火,以及稳定化退火、磁场退火等等。
1、金属工具使用时因受热而失去原有的硬度。
2、把金属材料或工件加热到一定温度并持续一定时间后,使缓慢冷却。退火可以减低金属硬度和脆性,增加可塑性。也叫焖火。
退火的一个最主要工艺参数是最高加热温度(退火温度),大多数合金的退火加热温度的选择是以该合金系的相图为基础的,如碳素钢以铁碳平衡图为基础(图1)。各种钢(包括碳素钢及合金钢)的退火温度,视具体退火目的的不同而在各该钢种的Ac3以上、Ac1以上或以下的某一温度。各种非铁合金的退火温度则在各该合金的固相线温度以下、固溶度线温度以上或以下的某一温度。
重结晶退火 应用于平衡加热和冷却时有固态相变(重结晶)发生的合金。其退火温度为各该合金的相变温度区间以上或以内的某一温度。加热和冷却都是缓慢的。合金于加热和冷却过程中各发生一次相变重结晶,故称为重结晶退火,常被简称为退火。
这种退火方法,相当普遍地应用于钢。钢的重结晶退火工艺是:缓慢加热到Ac3(亚共析钢)或Ac1(共析钢或过共析钢)以上30~50℃,保持适当时间,然后缓慢冷却下来。通过加热过程中发生的珠光体(或者还有先共析的铁素体或渗碳体)转变为奥氏体(第一回相变重结晶)以及冷却过程中发生的与此相反的第二回相变重结晶,形成晶粒较细、片层较厚、组织均匀的珠光体(或者还有先共析铁素体或渗碳体)。退火温度在Ac3以上(亚共析钢)使钢发生完全的重结晶者,称为完全退火,退火温度在Ac1与Ac3之间 (亚共析钢)或Ac1与Acm之间(过共析钢),使钢发生部分的重结晶者,称为不完全退火。前者主要用于亚共析钢的铸件、锻轧件、焊件,以消除组织缺陷(如魏氏组织、带状组织等),使组织变细和变均匀,以提高钢件的塑性和韧性。后者主要用于中碳和高碳钢及低合金结构钢的锻轧件。此种锻、轧件若锻、轧后的冷却速度较大时,形成的珠光体较细、硬度较高;若停锻、停轧温度过低,钢件中还有大的内应力。此时可用不完全退火代替完全退火,使珠光体发生重结晶,晶粒变细,同时也降低硬度,消除内应力,改善被切削性。此外,退火温度在Ac1与Acm之间的过共析钢球化退火,也是不完全退火。
重结晶退火也用于非铁合金,例如钛合金于加热和冷却时发生同素异构转变,低温为 α相(密排六方结构),高温为 β相(体心立方结构),其中间是“α+β”两相区,即相变温度区间。为了得到接近平衡的室温稳定组织和细化晶粒,也进行重结晶退火,即缓慢加热到高于相变温度区间不多的温度,保温适当时间,使合金转变为β相的细小晶粒;然后缓慢冷却下来,使β相再转变为α相或α+β两相的细小晶粒。
等温退火 应用于钢和某些非铁合金如钛合金的一种控制冷却的退火方法。对钢来说,是缓慢加热到 Ac3(亚共析钢)或 Ac1(共析钢和过共析钢)以上不多的温度,保温一段时间,使钢奥氏体化,然后迅速移入温度在A1以下不多的另一炉内,等温保持直到奥氏体全部转变为片层状珠光体(亚共析钢还有先共析铁素体;过共析钢还有先共析渗碳体)为止,最后以任意速度冷却下来(通常是出炉在空气中冷却)。等温保持的大致温度范围在所处理钢种的等温转变图上A1至珠光体转变鼻尖温度这一区间之内(见过冷奥氏体转变图);具体温度和时间,主要根据退火后所要求的硬度来确定(图2)。等温温度不可过低或过高,过低则退火后硬度偏高;过高则等温保持时间需要延长。钢的等温退火的目的,与重结晶退火基本相同,但工艺操作和所需设备都比较复杂,所以通常主要是应用于过冷奥氏体在珠光体型相变温度区间转变相当缓慢的合金钢。后者若采用重结晶退火方法,往往需要数十小时,很不经济;采用等温退火则能大大缩短生产周期,并能使整个工件获得更为均匀的组织和性能。等温退火也可在钢的热加工的不同阶段来用。例如,若让空冷淬硬性合金钢由高温空冷到室温时,当心部转变为马氏体之时,在已发生了马氏体相变的外层就会出现裂纹;若将该类钢的热钢锭或钢坯在冷却过程中放入700℃左右的等温炉内,保持等温直到珠光体相变完成后,再出炉空冷,则可免生裂纹。
含β相稳定化元素较高的钛合金,其β相相当稳定,容易被过冷。过冷的β相,其等温转变动力学曲线(图3)与钢的过冷奥氏体等温转变图相似。为了缩短重结晶退火的生产周期并获得更细、更均匀的组织,亦可采用等温退火。
均匀化退火 亦称扩散退火。应用于钢及非铁合金(如锡青铜、硅青铜、白铜、镁合金等)的铸锭或铸件的一种退火方法。将铸锭或铸件加热到各该合金的固相线温度以下的某一较高温度,长时间保温,然后缓慢冷却下来。均匀化退火是使合金中的元素发生固态扩散,来减轻化学成分不均匀性(偏析),主要是减轻晶粒尺度内的化学成分不均匀性(晶内偏析或称枝晶偏析)。均匀化退火温度所以如此之高,是为了加快合金元素扩散,尽可能缩短保温时间。合金钢的均匀化退火温度远高于Ac3,通常是1050~1200℃。非铁合金锭进行均匀化退火的温度一般是“0.95×固相线温度(K)”,均匀化退火因加热温度高,保温时间长,所以热能消耗量大。
球化退火 只应用于钢的一种退火方法。将钢加热到稍低于或稍高于Ac1的温度或者使温度在A1上下周期变化,然后缓冷下来。目的在于使珠光体内的片状渗碳体以及先共析渗碳体都变为球粒状,均匀分布于铁素体基体中(这种组织称为球化珠光体)。具有这种组织的中碳钢和高碳钢硬度低、被切削性好、冷形变能力大。对工具钢来说,这种组织是淬火前最好的原始组织。
球化退火的具体工艺(图4)有:①普通(缓冷)球化退火(图4a),缓冷适用于多数钢种,尤其是装炉量大时,操作比较方便,但生产周期长;②等温球化退火(图4b),适用于多数钢种,特别是难于球化的钢以及球化质量要求高的钢(如滚动轴承钢);其生产周期比普通球化退火短,不过需要有能够控制共析转变前冷却速率的炉子;③周期球化退火(图4c),适用于原始组织为片层状珠光体组织的钢,其生产周期也比普通球化退火短,不过在设备装炉量大的条件下,很难按控制要求改变温度,故在生产中未广泛采用;④低温球化退火(图4d),适用于经过冷形变加工的钢以及淬火硬化过的钢(后者通常称为高温软化回火);⑤形变球化退火,形变加工对球化有加速作用,将形变加工与球化结合起来,可缩短球化时间。它适用于冷、热形变成形的钢件和钢材(如带材)(图4e是在Acm或Ac3与Ac1之间进行短时间、大形变量的热形变加工者;图4f是在常温先予以形变加工者;图4g是利用锻造余热进行球化者)。
再结晶退火 应用于经过冷变形加工的金属及合金的一种退火方法。目的为使金属内部组织变为细小的等轴晶粒,消除形变硬化,恢复金属或合金的塑性和形变能力(回复和再结晶)。若欲保持金属或合金表面光亮,则可在可控气氛的炉中或真空炉中进行再结晶退火。
去除应力退火 铸、锻、焊件在冷却时由于各部位冷却速度不同而产生内应力,金属及合金在冷变形加工中以及工件在切削加工过程中也产生内应力。若内应力较大而未及时予以去除,常导致工件变形甚至形成裂纹。去除应力退火是将工件缓慢加热到较低温度(例如,灰口铸铁是500~550℃,钢是500~650℃),保温一段时间,使金属内部发生弛豫,然后缓冷下来。应该指出,去除应力退火并不能将内应力完全去除,而只是部分去除,从而消除它的有害作用。
还有一些专用退火方法,如不锈耐酸钢稳定化退火;软磁合金磁场退火;硅钢片氢气退火;可锻铸铁可锻化退火等。
--------------------------------------------------------------------------------
退火 annealing
将工件加热到预定温度,保温一定的时间后缓慢冷却的金属热处理工艺。退火的目的在于:①改善或消除钢铁在铸造、锻压、轧制和焊接过程中所造成的各种组织缺陷以及残余应力,防止工件变形、开裂。②软化工件以便进行切削加工。③细化晶粒,改善组织以提高工件的机械性能。④为最终热处理(淬火、回火)作好组织准备。常用的退火工艺有:①完全退火。用以细化中、低碳钢经铸造、锻压和焊接后出现的力学性能不佳的粗大过热组织。将工件加热到铁素体全部转变为奥氏体的温度以上30~50℃,保温一段时间,然后随炉缓慢冷却,在冷却过程中奥氏体再次发生转变,即可使钢的组织变细。②球化退火。用以降低工具钢和轴承钢锻压后的偏高硬度。将工件加热到钢开始形成奥氏体的温度以上20~40℃,保温后缓慢冷却,在冷却过程中珠光体中的片层状渗碳体变为球状,从而降低了硬度。③等温退火。用以降低某些镍、铬含量较高的合金结构钢的高硬度,以进行切削加工。一般先以较快速度冷却到奥氏体最不稳定的温度,保温适当时间,奥氏体转变为托氏体或索氏体,硬度即可降低。④再结晶退火。用以消除金属线材、薄板在冷拔、冷轧过程中的硬化现象(硬度升高、塑性下降)。加热温度一般为钢开始形成奥氏体的温度以下50~150℃ ,只有这样才能消除加工硬化效应使金属软化。⑤石墨化退火。用以使含有大量渗碳体的铸铁变成塑性良好的可锻铸铁。工艺操作是将铸件加热到950℃左右 ,保温一定时间后适当冷却 ,使渗碳体分解形成团絮状石墨。⑥扩散退火。用以使合金铸件化学成分均匀化,提高其使用性能。方法是在不发生熔化的前提下 ,将铸件加热到尽可能高的温度,并长时间保温,待合金中各种元素扩散趋于均匀分布后缓冷。⑦去应力退火。用以消除钢铁铸件和焊接件的内应力。对于钢铁制品加热后开始形成奥氏体的温度以下100~200℃,保温后在空气中冷却,即可消除内应力。
退火
为了消除塑料制品的内应力或控制结晶过程,将制品加热到适当的温度并保持一定时间,而后慢慢冷却的操作。
--------------------------------------------------------------------------------
退火 annealing
加热使DNA双螺旋解开,在一定的条件下,两条互补的单链依靠彼此的碱基配对重新形成双链DNA的过程,亦即复性过程。热变性的DNA单链在缓慢冷却过程中可以达到很好的退火。退火的两条单链可以来自同一个双链的DNA分子,也可以来自不同的DNA分子。退火是变性的逆转过程,它受温度、时间、DNA浓度、DNA顺序的复杂性等因素的影 响。如PCR反应中引物与模板DNA的退火,核酸杂交中探针与被检DNA的退火
与“annealing,退火”相关的词条
→如果您认为本词条还有待完善,请 编辑词条
词条内容仅供参考,如果您需要解决具体问题
(尤其在法律、医学等领域),建议您咨询相关领域专业人士。
0

同义词: 暂无同义词
关于本词条的评论 (共0条)发表评论>>